July 2025
NewsletterSummary:
-
-
- The Chikungunya virus is increasingly spreading to Central Europe via the Asian tiger mosquito – first autochthonous case reported in the Alsace region
- Key symptoms include high fever and severe joint and muscle pain; up to 50% of patients may develop chronic arthritis
- PCR diagnostics are most effective within the first 5 days after symptom onset, serological detection from day 8-10
- Two vaccines have been approved in the EU: live attenuated vaccine Ixchiq® (2024) and inactivated vaccine VIMKUNYA™ (2025)
- Vaccination is recommended for travel to outbreak areas, extended stays in endemic regions, and occupationally exposed individuals
-
With the summer and holiday months, the occurrence of travel-associated infectious diseases naturally increases. However, locally emerging diseases transmitted by vectors active during summer are also becoming more prevalent. Due to climatic changes, various vectors are expanding their habitat northward, increasing the risk of local transmission of pathogens from the arbovirus group (arthropod-borne), which were traditionally more associated with travel.
Pathogen
The causative agent of Chikungunya fever is the virus of the same name, CHIKV for short, an RNA virus from the genus Alphavirus of the Togaviridae family. The primary vectors are mosquitoes of the species Aedes aegypti (yellow fever mosquito) and, following recent adaptation of the virus, also Aedes albopictus (Asian tiger mosquito). Of these mosquitoes, originally native to the tropics and subtropics, the latter has spread reliably over recent years from the Mediterranean region to Central Europe.
Autochthonous infections, i.e., locally acquired infections, are therefore becoming more likely in the future. While regularly reported for Southern France and Italy since 2007, this month marks the first report of a highly probable autochthonous Chikungunya case in Central Europe (Alsace region). Although not yet highly likely at present, a possible spread to Austria must be anticipated in the future.
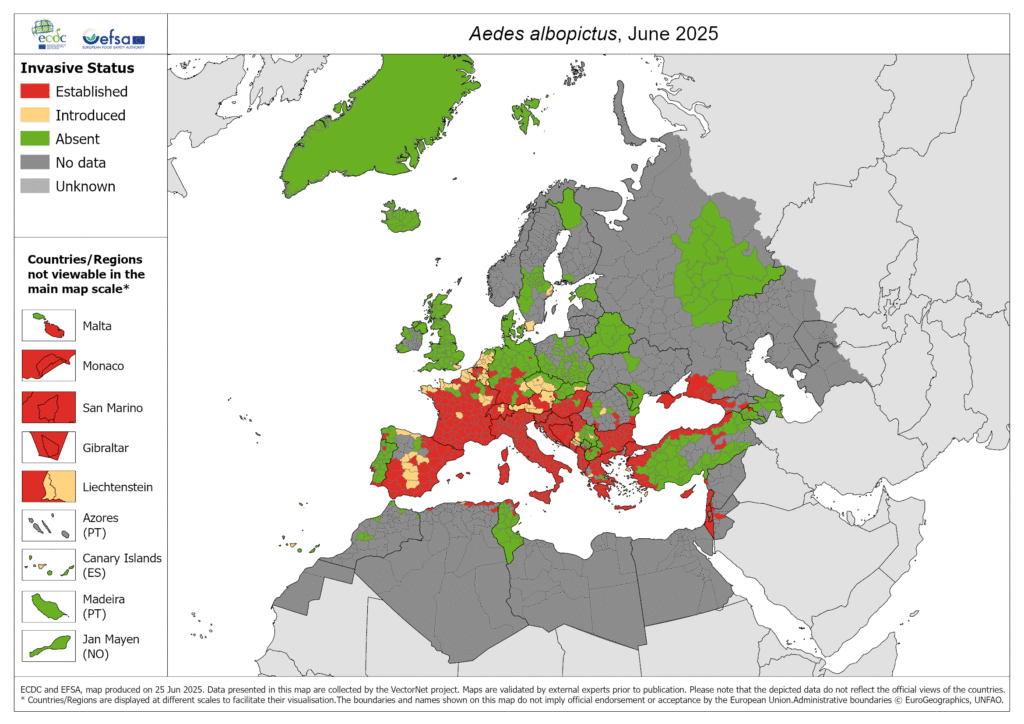
Figure 1: Spread of the asian tiger mosquito as of June 2025 (ECDC)
Clinical Symptoms
The incubation period is usually between 3 – 7 days. In addition to nonspecific symptoms such as high fever and occasional maculopapular rash, severe muscle and symmetrical joint pain are the cardinal symptoms. The name Chikungunya is based on the presentation of these joint complaints, meaning “the one who walks bent over” in a Tanzanian language.
In many cases, the disease heals within two weeks without lasting damage, followed by lifelong immunity. However, according to various studies, up to 50% of patients may develop post-acute (up to 3 months) as well as chronic (> 3 months) forms of arthritis.
Furthermore, CHIKV infections can also lead to neurological manifestations, such as encephalitis, meningitis, or Guillain-Barré syndrome. Newborns, elderly individuals, and people with pre-existing conditions (diabetes, cardiovascular diseases, and liver or kidney dysfunction) have a higher risk of severe disease progression.
Diagnostics
Within the first 5 days after symptom onset, PCR detection from blood is diagnostically effective. Serological detection is recommended from day 8-10 onwards.
At our institute, we offer Chikungunya PCR for suspected travel-associated infection as well as for differential diagnosis of unclear arthralgias. Unfortunately, the costs for this PCR are not yet covered by health insurance.
Vaccination Recommendations
Two vaccines against Chikungunya were recently approved in the European Union. An attenuated live vaccine Ixchiq® (2024) and an inactivated vaccine with Virus-like Particle (VLP) design VIMKUNYA™ (2025). In a recent communication from the STIKO of the RKI, the following recommendations were issued:
1. Recommendation as Travel Vaccination
-
- generally recommended for travel to current outbreak areas
- recommended for travel to known endemic areas for stays > 4 weeks or repeated short trips
2. Recommendation for Occupational Indication
-
- activities with CHIKV exposure (laboratories)
- occupational travel to endemic areas with increased vector exposure
3. Notes for Special Population Groups
-
- Live vaccine Ixchiq® is contraindicated in individuals >65 years, pregnancy, breastfeeding, and immunodeficiency
- Inactivated vaccine VIMKUNYA™ can be used in patients > 65 years and is recommended for single trips due to its enhanced safety profile compared to the live vaccine
Primary immunization is achieved through a single dose. No data on booster recommendations are currently available.
Links:
Dr.med. Guido Wollmann
guido.wollmann@i-med.ac.at
+43 512 9003 71742



